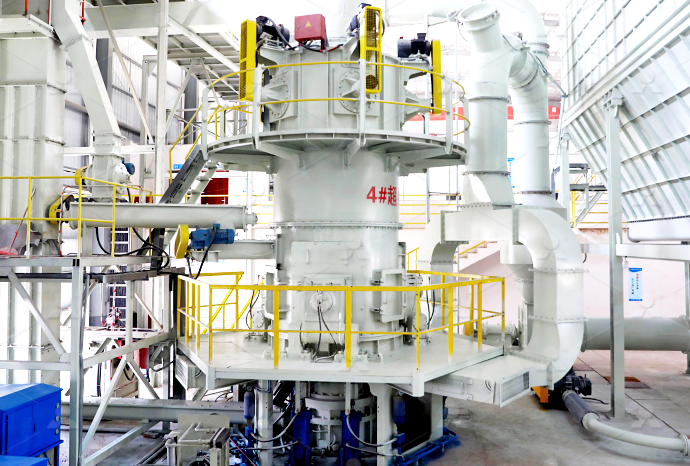
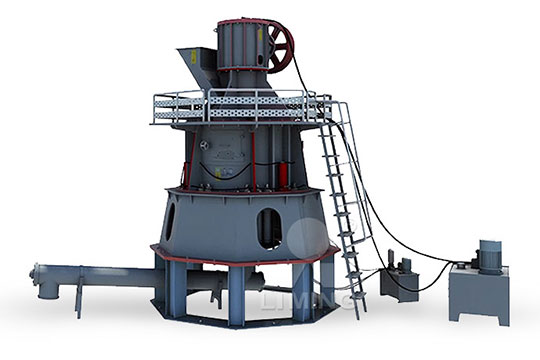
Sodium hydroxide complete equipment
.jpg)
Caustic (Sodium Hydroxide) Chemical Pumps Arroyo Process
Handle sodium hydroxide with advanced chemical pumps supplied by Arroyo Process Equipment Explore transfer, metering, and feed pumps built for caustic solutions in chemical and industrial processesFor over 30 years, SCG Process has been suppling complete engineered systems that are factory assembled and tested for the liquid chemical feed of Caustic Soda (Sodium Hydroxide) Our metering pump systems are easy to Caustic Soda / Sodium Hydroxide SCG ProcessOur Sodium Hydroxide (NaOH) Dilution System is designed for the accurate dilution and highvolume transfer of concentrated sodium hydroxide solution into railcars, tank trucks, and storage tanks As an alternative to diluting with Sodium Hydroxide Dilution System Skid MountedOur Caustic Soda Manufacturing Equipment is a comprehensive solution designed to facilitate the efficient production of highquality caustic soda (sodium hydroxide) Send Inquiry DescriptionChina Caustic Soda Manufacturing Equipment

[Hot Item] Caustic Soda Sodium Hydroxide Equipment
Compared to dissepiment method and hydrolyzing method, this equipment adopts ionic membrane to produce caustic which as the most advanced technology on the global, has many advantages including lower consumption of power (32% The complete sodium hydroxide production plant uses forced recirculation monopolar membrane technology In addition to the core electrolysis process, brine preparation, chlorine gas recovery and anhydrous caustic soda production 27,000 TPY Caustic Soda Plant for Sale at Phoenix All the equipment is still in excellent running condition This complete anhydrous soda caustic plant uses raising film evaporation and falling film concentration process to produce contracted liquid and/or flake sodium hydroxide products 180,000 TPY Anhydrous Caustic Soda (NaOH) for Sale Our Safe Handling Guide provides an overview of the essentials of Sodium Hydroxide safety Liquid Sodium Hydroxide, sometimes called Caustic Soda, is most commonly made via the electrolysis of brine (Sodium Chloride) Brine Safe Handling Guide: Sodium Hydroxide CORECHEM
.jpg)
Sodium hydroxide making machine
Buy wholesale sodium hydroxide making machine that is trustworthy during a power outage in your home or at work Visit Alibaba and order the right Gas Generation EquipmentOnly properly trained and equipped personnel should be permitted to handle sodium hydroxide Operators should wear approved PPE equipment including impervious clothing, footwear, gloves, goggles and a respirator Sodium Sodium hydroxide (NaOH) Handling Design, Loading, 2012年11月7日 Sodium hydroxide (pellets) 1 Date: 11/7/2012 SOP Template developed by The UC Center for Laboratory Safety Standard Operating Procedure Sodium Hydroxide This SOP is not complete until it has been signed and dated by the PI and relevant lab personnel Personal Protective Equipment (PPE)Standard Operating Procedure UC Santa BarbaraSodium Hydroxide CCOHS Chemical Profiles Sodium Hydroxide On this page What are other names or identifying information for sodium hydroxide? protective equipment requirements and personal hygiene measures are being followed Only Sodium Hydroxide Chemical Profiles Canadian Centre for
.jpg)
3% SODIUM HYDROXIDE
Sodium hydroxide (CASNo) 0 – 5 Met Corr 1, H290 Acute Tox 4 (Dermal), H312 Special protective equipment and precautions for firefighters firefighting : Do not attempt to take action without suitable protective equipment Selfcontained breathing apparatus Complete protective clothing SECTION 6: Accidental release In the United States, Sodium Hydroxide is a “tightfill” (closedloop) loading operation and is loaded or unloaded into rail cars via chemical hoses, 3” stainless steel or PTFE lined loading arms Sodium hydroxide, if not handled properly can cause serious injuries and Personal Protective Equipment (PPE) is requiredSodium hydroxide (NaOH) Handling Design, Loading, andCaustic soda, Lye [Sodium hydroxide], Soda lye, Sodium hydrate CAS No RTECS No WB DOT ID Guide 1823 154(dry, solid) 1824 154(solution) Formula NaOH Conversion IDLH 10 mg/m 3 See: Exposure Limits NIOSH REL C 2 mg/m 3 OSHA PEL TWA 2 mg/m 3 See Appendix G Measurement MethodsCDC NIOSH Pocket Guide to Chemical Hazards Sodium hydroxide2023年6月18日 Caustic soda, also known as sodium hydroxide (NaOH), is a highly versatile and essential chemical compound used in various industries such as chemical manufacturing, pulp and paper production, textiles, soaps and detergents, and water treatment The production and supply of caustic soda play a crucial role in Nigeria and Africa's industrial development In How To Start A Lucrative Caustic Soda (Sodium Hydroxide)

Sodium Hydroxide Suppliers Australia Australian Chemical
We have compiled a list of Sodium Hydroxide suppliers in Silform suppliers of chemical reagents, biological materials and equipment View our best online prices, ABN required to order Silform SODIUM HYDROXIDE LR Minipearl Quick enquiry Where to buy Suppliers range: 500g Pack This will fizz in water until the reaction is complete, This Standard Operating Procedure (SOP) summarizes the safe handling of sodium hydroxide Sodium hydroxide is a strong base that will be used to make an electrolyte Specific steps are outlined for weighing, dissolving, and storing sodium hydroxide Personal protective equipment including gloves, goggles, and a lab coat are required due to sodium hydroxide being highly Standard Operating Procedure (SOP) For ( Sodium HydroxideNitric acid + sodium hydroxide → sodium nitrate + water Acid + alkali → salt + water Symbol equations A symbol equation describes a reaction more precisely using chemical symbols and formulasWhat is a neutralisation reaction? BBC BitesizeUse microscale titration to complete an acid–base neutralisation with sodium hydroxide in this class practical Includes kit list and safety instructions Use microscale titration to complete an acid–base neutralisation with sodium hydroxide in this class practical Equipment Apparatus Graduated glass pipette, 2 cm 3;A microscale acid–base titration Experiment RSC Education

SAFETY DATA SHEET: SODIUM HYDROXIDE 25% Integra Clear
Wear full protective equipment, including a complete protective suit and an appropriate respirator depending upon the size of the spill Absorb with sand or inert material Sweep or scoop into a disposal container Sodium hydroxide LC50 Mosquito fish: 125 mg/L 96h; EC50 No information available Persistence and degradability: 2024年3月18日 Sodium Chlorate Safety Data Sheet, Sodium Hydroxide Revision No G 03/18/2024 Page 5 18 SECTION 3 COMPOSITION, INFORMATION OR INGREDIENTS (Continued) SECTION 4 FIRST AID MEASURES CAS Number Percentage VOL WT ND 0 030 Name Chloric Acid, Sodium Salt Exposure Limits PEL TLV Not Established Not Sodium Hydroxide All Grades Kuehne CompanyArroyo Process Equipment provides chemical transfer pumps like the Viking 327A Series to facilitate the safe and efficient transfer of sodium hydroxide from storage tanks to processing units Our pumps are designed to handle a variety of viscosities, ensuring smooth and reliable performance regardless of concentrationCaustic (Sodium Hydroxide) Chemical Pumps Arroyo Process EquipmentLearn how the amounts of chemicals in solution are measured with Bitesize GCSE Combined Science (OCR 21C)How are the amounts of chemicals in solution measured?

Required Practical 4 Physics Maths Tutor
Test for group 2 ions: sodium hydroxide Method Accuracy Explanation 1Place 10 drops of 01 moldm 3 barium chloride in a clean test tube Must be clean to ensure a clear test result 2Add 10 drops of 06 moldm 3 sodium hydroxide solution, mixing well and recording any observationsExactly 5000 mL of HCl is required to neutralise 2000 mL of a 00500 mol/L solution of sodium carbonate In this example, the titrant is the HCl solution and the titre volume is 5000 mL Therefore, Volumetric flask rinsed with distilled water; Pipette rinsed with sodium carbonate solution (standard) Conical flask rinsed with distilled waterTitration: Standard Solution, Washing, Setup – HSC Chemistry2024年11月14日 Titration of sodium hydroxide with hydrochloric acid general remarks Determination of sodium hydroxide concentration is about as often discussed as hydrochloric acid titration both acid and base are strong, so calculation of titration curve and equivalence point are pretty straightforward However, titration itself is not as easyTitration of sodium hydroxide with hydrochloric acidSodium Hydroxide, 01M Page 1 of 4 Sodium Hydroxide, 01M Section 1 Product Description Product Name: Sodium Hydroxide, 01M Recommended Use: Science education applications Synonyms: Soda Lye, Sodium Hydroxide 01N, Caustic Soda, Lye Distributor: Carolina Biological Supply Company 2700 York Road, Burlington, NC 27215 18002271150Sodium Hydroxide, 01M Safety Data Sheet Taylor Made Science

174: Titrations and pH Curves Chemistry LibreTexts
2020年8月14日 At the equivalence point (when 250 mL of \(\ce{NaOH}\) solution has been added), the neutralization is complete: only a salt remains in solution (NaCl), and the pH of the solution is 700 Adding more \(\ce{NaOH}\) produces a rapid increase in pH, but eventually the pH levels off at a value of about 1330, the pH of 020 M \(NaOH\)2018年8月3日 The CL10HY Density Cell in conjunction with the Series 2000 Density Digital Converter can measure the temperature and density of a caustic soda (sodium hydroxide) process stream The Series 2000 then processes this information Once the information is processed the density is calculated at a reference temperature The specific gravity, baumé Meas Control Equipment Applications Sodium Hydroxide2023年11月10日 Using the appropriate protective equipment is essential to ensure your safety when working with sodium hydroxide Here is the necessary gear: Chemicalresistant gloves : As mentioned earlier, wearing chemicalresistant gloves is vital to protect your hands from direct contact with sodium hydroxideBenefits And Risks Of Sodium Hydroxide In Soap Complete Guide2020年7月7日 In this practical students measure the temperature change during the neutralisation of hydrochloric acid and sodium hydroxide solution Students should be able to calculate the mean maximum temperature to complete Temperature change of neutralisation – practical

Understanding AcidAlkali Titrations: Methods
2022年6月23日 The volume of hydrochloric acid needed to neutralise the sodium hydroxide is then used to calculate the concentration of the sodium hydroxide, as shown in the following worked example Example – processing Caustic Soda Sodium Hydroxide Equipment, The Corporation has delivered a number of complete plants that are technically advanced, highefficiency, easy to be operated, and cooperated with many international groups such as PG, Unilever, Henkel, etc[Hot Item] Caustic Soda Sodium Hydroxide EquipmentTitration equipment A titration is a very commonly used type of quantitative analysis {1}\) was added from the burette into a conical flask containing 20 cm 3 of sodium hydroxide solution Titration equipment Chemical analysis National 5 Chemistry1 A total volume of 50 cm3 of hydrochloric acid is added gradually to 50 cm3 of sodium hydroxide solution containing some universal indicator Complete the table, giving all values to the nearest 005 cm3 (3) burette reading at end in cm3 burette reading at start in cm3Acids, Alkalis and Titrations IG Exams

Fiche complète pour Sodium, hydroxyde de CNESST
Sodium hydrate; Sodium hydroxide; White caustic; Commentaires 1 Les principales impuretés de l'hydroxyde de sodium comprennent : chlorure de sodium, carbonate de sodium, sulfate de sodium, chlorate de sodium, potassium et des métaux tels : fer et nickelThe complete balanced equation for the reaction between sulfuric acid and sodium hydroxide is: H 2 SO 4 + 2NaOH → Na 2 SO 4 + 2H 2 O Thus, writing this in a full ionic reaction form , we get: 2H + + SO 4 2+ 2 Na + + 2OH – → 2Na + + SO 4 2+ 2H 2 O So, the net ionic equation of sulfuric acid and sodium hydroxide, after crossing out Titration of Sulfuric Acid and Sodium HydroxideTo improve magnesium reduction, which also improves silica reduction in cold process softening, sodium aluminate may be used The sodium aluminate provides hydroxyl ion (OH) needed for improved magnesium reduction, without increasing calcium hardness in the treated waterIn addition, the hydrolysis of sodium aluminate results in the formation of aluminum hydroxide, Water Handbook Precipitation Softening VeoliaSafety Emergency Preparedness Eye protection is mandatory in this lab, and you should not wear shorts or open toed shoes; The following image shows the damage 4 M sodium hydroxide can cause By following proper procedures and using proper PPE (Personal Protective Equipment) the risk to damage can be reduced to near zero72: Lab Titrations Chemistry LibreTexts
.jpg)
718: Titration Experiment Chemistry LibreTexts
In the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1 \[\ce{HCl} \left( aq \right) + \ce{NaOH} \left( aq \right) \rightarrow \ce{NaCl} \left( aq \right) + \ce is the point in a neutralization 2022年4月22日 Standard solutions of sodium hydroxide are not stored in glass bottles as NaOH reacts The complete titration curve is shown in Figure 9215 b Figure 9215 concerns about analysis time are less significant When 92: Acid–Base Titrations Chemistry LibreTextsSodium hydroxide (NaOH) is the least expensive and most commonly used alkaline component in the smallscale biodiesel product ion process equipment and materials you need to complete a batch 1 liter of new vegetable (cooking) Biodiesel: DoItYourself Production Basics – ATTRA – Materials and Equipment Materials: warm olive oil (preheated by instructor), 9 M sodium hydroxide solution, food coloring, assorted fragrances, stearic acid Equipment: tall 250 mL beaker, PLASTIC stirring rod, glass pipets and pipet bulbs Your instructor has a beaker of olive oil, preheated to 35°C, at the front bench12: Making Soap Saponification (Experiment Chemistry
.jpg)
EXPERIMENT 12 A: STANDARDIZATION OF A SODIUM HYDROXIDE
Sodium hydroxide is deliquescent (absorbs moisture from the atmosphere) solid It cannot be weighed accurately Therefore, it is not possible to prepare a standard solution of sodium hydroxide of accurately known concentration by weighing NaOH A sodium hydroxide solution of approximate concentration (02 M) is to be prepared It is then The above reactions indicate that demineralization completely removes the cations and anions from the water In reality, because ion exchange reactions are equilibrium reactions, some leakage occurs Most leakage from cation units is sodium This sodium leakage is converted to sodium hydroxide in the anion unitsIon Exchange Water Demineralization Handbook Veolia2022年10月14日 In many paper making processes, wood is treated with a solution containing sodium sulfide and sodium hydroxide This helps dissolve most of the unwanted material in the wood, leaving relatively pure cellulose, which forms the basis of paper In the paper recycling process, sodium hydroxide is used to separate the ink from the paper fibers allowing the paper Sodium Hydroxide Chemical Safety FactsSafety Data Sheet SODIUM HYDROXIDE SDS no FB4MH9MJ • Version 10 • Date of issue: SECTION 1: Identification GHS Product identifier Product name SODIUM HYDROXIDE Other means of identification Product Name Product Code Sodium Hydroxide AR Minipearl SA000 Sodium Hydroxide Pellet ARl SA178 Sodium Hydroxide LR Minipearll SL000Safety Data Sheet SODIUM HYDROXIDE
.jpg)
Neutralisation (titration) AQA Required Practical
Fill a burette with the sodium hydroxide solution, then add a few drops of indicator into the conical flask Slowly add the alkali to the flask and swirl the flask whilst looking for a colour change Once a sudden colour change has occurred, stop adding alkali, and record the volume of alkali added from the buretteAim To standardize a sodium hydroxide (NaOH) solution against a primary standard acid [Potassium Hydrogen Phthalate (KHP)] using phenolphthalein as an indicator Variables Equipment 2 g of KHPTwo 100 cm3 Beakers (One for making the KHP solution, one for pouring NaOH into the burette)1 Digital Balance (up to 2 decimal places accuracy)1 Stirring rod1 Titration Lab: NaOH with Standardized solution of KHP