Which powder making process of quicklime
.jpg)
How to Make Quicklime: 10 Steps (with Pictures)
2024年4月16日 Quicklime, also known as calcium oxide (CaO), is a caustic alkaline substance It has been used by humans for centuries for many things 2023年2月4日 Lime, also known as quicklime or burnt lime, is mainly composed of calcium oxide, molecular formula CaO, which is a white block or powder cubic crystal The lime commonly used in industry will be dark gray due to impurities Lime/quicklime for metallurgy – how producing and When water is to powdered quicklime and the resulting mixture is subsequently placed in a kiln or an oven, and then pulverised with water, the product formed is hydrated lime The resulting Quicklime Preparation, Properties, and Applications with FAQsQuicklime is a caustic compound known by its chemical name calcium oxide (CaO) It appears as a white powder when cold, and yellow when heated Quicklime is produced by heating What is Quicklime and How is it Made? Science Struck

Quick Lime Preparation, Properties and Uses Hebei Yayang
2023年10月11日 To simplify, hydrated lime is the result of adding water to powdered quicklime, putting it in a kiln or oven, and then pulverizing it with water The resulting lime has a density of The heating process causes a chemical reaction called calcination and this produces calcined lime also known as quicklime The quicklimes, after cooling down, are used in various differing Making LimeThe major use of quicklime is in the basic oxygen steelmaking (BOS) process Its usage varies from about 30 to 50 kilograms (65–110 lb) per ton of steel The quicklime neutralizes the acidic oxides, SiO 2 , Al 2 O 3 , and Fe 2 O 3 , to Calcium oxide Wikipedia2024年6月23日 QuickLime, also known as calcium oxide, is a key ingredient with a wide range of applications across various industries Understanding its properties and uses can provide valuable insights into its significance Key QuickLime 101: Everything About This Super Substance

Quicklime (Calcium Oxide) CaO, Kemicalinfo
The most common method for synthesizing quicklime is by heating limestone to a high temperature of about 9001000°C in a lime kiln This process, known as calcination, releases There are three main ways of slaking the Quicklime: in an excess of water to produce a putty; in a shortfall of water to produce a powder hydrated or bag lime; in damp sand to produce a hot mix Lime carbonation Lime sets by Lime and its ProductionLearn about Quicklime topic of Chemistry in details explained by subject experts on Vedantu Register free for online tutoring session to clear your doubts It is a shapeless white amorphous powder with high melting and boiling points, Used in making porcelain and glass In preparation of bleaching powder, calcium carbide, Quicklime Properties, Uses and Application Vedantu2023年10月11日 How do you determine whether to use hydrated lime or quicklime? If dry, the process feed rate determines the choice between using hydrated or quicklime Just remember that quicklime is more “reactive” than hydrated lime However, in some processes, hydrated lime is not suitable even if we inject it dry, and vice versa with quicklimeQuick Lime Preparation, Properties and Uses Hebei Yayang

Making Lime
Quicklime When a calcium limestone or chalk rock, that comprises mainly of calcium carbonate (CaCO 3), is heated in a kiln, it changes by a process called calcination into quicklime also known as 'burnt lime' and chemically is mainly calcium oxide (CaO), and the calcination process releases a gas from the rock which is carbon dioxide (CO 2) Hydrated Lime2024年2月1日 Lime (CaO), also known as quicklime, is a white, crystalline, alkaline substance produced by heating limestone (calcium carbonate) pulverized limestone has been crushed and ground into a fine powder The pulverization process increases the surface area of the limestone particles, making it more reactive and suitable for various What Is Lime? Lime AssociationFrom the moment it is burnt the material starts to degrade by ‘airslaking’ Combining Quicklime (CaO) and water (H20) produces Calcium Hydroxide (Ca(OH)2 slaked lime and heat There are three main ways of slaking the Quicklime: in an excess of water to produce a putty; in a shortfall of water to produce a powder hydrated or bag lime;Lime and its Production2024年11月8日 quicklime (CaO), compound of one atom of calcium and one atom of oxygen that is a white or grayish white solid produced in large quantities by roasting calcium carbonate so as to drive off carbon dioxideAt room temperature, CaO will spontaneously absorb carbon dioxide from the atmosphere, reversing the reactionIt will also absorb water, converting itself into Quicklime Formula, Uses, Definition Britannica

Calcium Oxide (CaO) Preparation, Properties Uses of Quicklime
Calcium oxide, also known as quicklime, is an alkaline substance that has been in use since the medieval age It is believed that quicklime is one of the oldest chemicals known to the human race It can also be referred to as burnt lime or lime Table of Contents Preparation of Calcium Oxide; Structure of CaO Molecules; Lime Water FormulaBurnt Lime 40 is a white, lump calcium oxide product which is made by crushing and screening quicklime It is t ypically used as a flux in the steel making process and in the sugar refining industryQuicklime Singleton BirchHydrated lime, also known as slaked lime, is produced by adding water to quicklime in a controlled process It is a fine white powder that is commonly used in construction, agriculture, and water treatment Hydrated lime is less caustic and more stable than quicklime, making it Hydrated Lime vs Quicklime What's the Difference? This vs That2024年2月23日 The limestone powder making process and its versatile applications highlight the importance of this finely ground material across multiple industries From construction to agriculture, environmental remediation, and various manufacturing sectors, limestone powder plays a crucial role in enhancing products and processes, contributing to sustainable and Introduction of limestone powder making process and application
.jpg)
Applications of Quicklime Hydrated Lime
and what remains is quicklime, calcium oxide Limestone decomposes into quicklime and carbon dioxide: CaCO3> CaO + CO2 by weight 100 > 56 + 44 The process is called ‘calcination’ If calcination is carried out correctly the lumps of quicklime are approximately the same size as the original lumps of limestone but much less dense, becauseShop high calcium quicklime online Perfect for making lime putty hotmixed lime mortars Available as a fine powder or as pebbles from 515mm However the mixing process can take more time than alternatives and due to the heat Buy Quicklime Online Powder Pebble Cornish LimeQuicklime is used in a wide variety of applications, including the manufacture of iron and steel, the manufacture of paper and pulp, the treatment of water and flue gases and the mining industry In the basic oxygen steelmaking (BOS) process, quicklime Quicklime Preparation, Properties, and Applications with FAQs2023年7月19日 In the lime production process, limestone is heated at high temperatures, which causes it to decompose into calcium oxide (quicklime) and carbon dioxide This process, known as calcination, is a natural chemical reaction that has LIME: Everything you need to know to get started

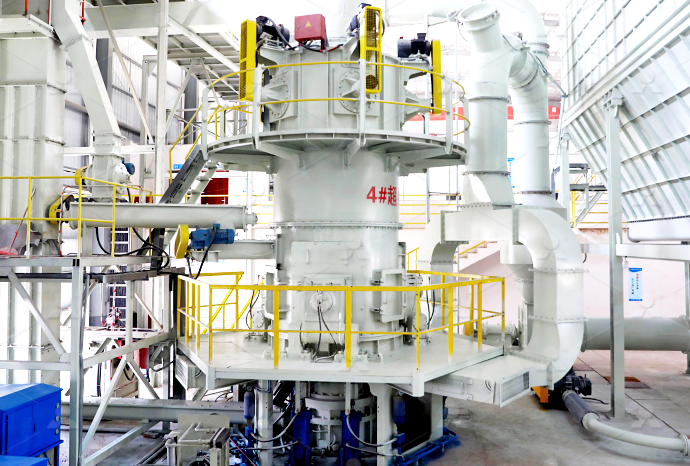
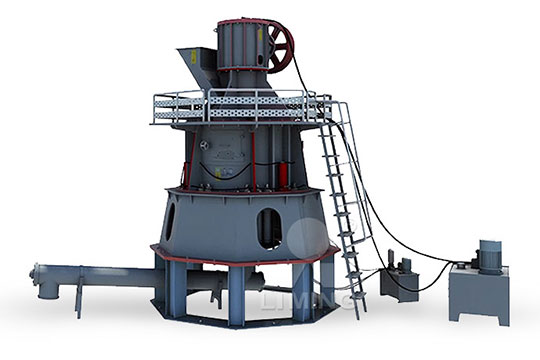
Type Selection of Quicklime Deep Processing Equipment
2022年12月22日 CLIRIK has been committed to the research and development of energysaving and environmentally friendly grinding equipment The vertical mills, highpressure mills, and micropowder mills developed and produced by CLIRIK have won high praise from users in quicklime processingCalcium Oxide Quicklime Calcium oxide (chemical formula: CaO), also called quicklime or burnt lime, is a widely used chemical compound in our daily lives formed by ionic bonding between one calcium atom and one oxygen atom The white or grayishwhite crystalline solid, calcium oxide can be produced in large quantities by driving off carbon dioxide from calcium carbonateCalcium Oxide Quicklime Chemical Formula, Uses2023年10月27日 Quicklime can be useful, but must be handled carefully to prevent an adverse reaction that can cause skin and eye burns as well as inhalation hazards for anyone making or working with it As such, workers must utilize full personal protective equipment including goggles, head hood, polyurethane or rubber gloves, and a cotton work suit with leather boots and dust Quicklime: A PrimerCalcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO 3; mineral calcite) in a lime kilnThis is accomplished by heating the material to above 825 °C (1,517 °F), [6] [7] a process called calcination or limeburning, to liberate a molecule of carbon dioxide (CO 2), leaving quicklime Calcium oxide Wikiwand

How Lime is Made
Quicklime can be processed into hydrated lime by crushing the quicklime, adding water to the crushed lime (water accounts for approximately 1% of raw hydrate), and then classifying the hydrated lime to ensure it meets customer specifications before it is transported Lime Basics2022年10月12日 Quicklime can be applied to the soil in a number of ways, including as a dry powder, in a slurry, or in pellet form Quicklime can also be used as a fertilizer It is a source of calcium, which is an essential nutrient for plants Quicklime can be applied to the soil in the same ways as it can be used as a soil amendmentThe Many Uses Of Quicklime – AccessibleGardensPure lime, or quicklime, is calcium oxide Its ease of manufacture and chemical properties make it an important industrial chemical Pure lime, or quicklime, It is one of the oldest types of mortar dating back to ancient times Nowadays, Lime – a timetested chemical — Science Learning Hub2013年3月3日 Quicklime: Quicklime is dangerous to handle and store because it is highly reactive It heats rapidly and undergoes a violent reaction when water is applied This process is called slaking If the water is applied slowly and in Understanding Lime: an introduction to forms of lime
.jpg)
Quicklime (Calcium Oxide) CaO, Kemicalinfo
The most common method for synthesizing quicklime is by heating limestone to a high temperature of about 9001000°C in a lime kiln This process, known as calcination, releases carbon dioxide and leaves behind quicklime Processing and grinding the quicklime produces a fine powder for multiple applicationsCalbux 90 from Buxton Lime25kg plastic bags Quicklime powder used for making Hot Lime and Lime Putty WARNINGQuicklime is a highly reactive material which can be dangerous if not handled correctly The Slaking process (Mixing quicklime with water) generates extremely high temperatures Please make sure you understand the risks and are aware of the correct safety Quicklime carringtonlimeAccording to the quicklime mesh and output, the following suggestions are given for the selection of quicklime powder grinding mills for reference only Quicklime Powder 1 Raymond mill is used for quicklime powder making It is recommended to use Raymond mill for the processing of quicklime with an output of 1 to 9 tons and a mesh of 80 to How to choose quicklime powder grinding mill?Phosphorus removal: Phosphorus, contained in the iron ore and the scrap metal that are used to start the steelmaking process, can seriously damage the properties of steelIn large quantities, it lowers the ductility of the steel making it easy to fracture when it is coldworked Quicklime added to the metalmaking process extracts the phosphorus in the steel, lowering its proportion to Lime, an essential component in the steel industry

Quicklime Supplier, Price, Specification SteelScope
Calcium oxide, also called quicklime or burnt lime, is a versatile chemical compound widely utilized in various industries At room temperature, it appears as a white, caustic, alkaline, crystalline solid The primary application of quicklime is in the basic oxygen steelmaking (BOS) process, where it plays a crucial rolequick lime, is an exothermic process releasing a great quantity of heat This hydration process when done with just the right amount of water is called “Dry Hydration” In this case the hydrate material is a dry powder If excess water is used for hydration, the process is called “Slaking” In this case, the resultant hydrate is in aAn Overview of Lime Slaking and Factors That Affect the Process2023年9月27日 Quicklime plays an essential role in making steel Hydrated lime is another type of lime in the list of types of lime that is made by adding water to Quicklime This process creates a fine, It is available as a pure white powder When exposed to the atmosphere and in the presence of water, slaked lime absorbs carbonic acidDiscover the different types of Lime for your Construction Projectsusing a batch process However, if a continuous or semicontinuous process is used, then care must be taken that the mass of fuel used and the mass of quicklime produced correspond directly In our example, we will take a figure for the coal used as 200 kg/tonne of quicklime produced Figure 1: American Society of TestingHOW TO CALCULATE EFFICIENCY OF YOUR LIME BURNING PROCESS

Calcium Oxide Formula, Properties, Preparation and Uses
2024年7月30日 Calcium Oxide, also known as quicklime, lime water, or burnt lime, is a chemical compound with the formula CaO Its common name is quicklime Calcium oxide is composed of one calcium (Ca) atom and one oxygen (O) atom, with a chemical formula of CaO Calcium oxide has a high melting point of approximately 2,572 degrees Celsius (4,662 degrees If a precisely controlled amount of water is added to quicklime, a (violent) reaction ensues with much evolution of heat In the chemical reaction with water, the lumps of quicklime break down to a dry fine white powder known as hydrated lime or lime hydrate Quicklime + (a controlled amount of) water > hydrated lime (also called lime hydrate)Applications of Quicklime Hydrated Lime2023年7月31日 In the basic oxygen steelmaking (BOS) process, quicklime plays a crucial role Quicklime and hydrated lime can significantly increase the loadcarrying capacity of claycontaining soils by participating in chemical reactions with finely divided alumina and silica to create calcium silicates and aluminates, which have cementing propertiesQuicklime Preparation, Properties, Health Hazards, and 2024年4月9日 Finely ground quicklime (CaO) is added to standard bleaching powder to reduce water content further The quicklime absorbs any residual water present and is converted into calcium hydroxide Although this operation decreases the available chlorine content by 1–2%, the additional drying yields tropical bleach, which is stable up to temperatures of 100 °CBleaching Powder: Production and Uses

(PDF) The Effects of Limestone Characteristics and Calcination
2001年4月1日 This study has examined the effects of limestone characteristics (microstructure and texture) and calcination temperature on the reactivity of the produced quicklimeSlaked lime is obtained by the process of slaking, where quick lime is combined with water Slaked lime is also known as Hydrate of lime This is available as pure lime in the form of a white powder Slaked lime when exposed to the atmosphere, absorbs carbonic acid in What are the Types and Uses of Lime in Construction?High Calcium Quicklime Pebble and Powder, used for making lime putty and hotmixed or hotlime Mortars Quicklime is Limestone (a rock rich in calcium carbonate) that has undergone a chemical change in a kiln, liberating it of all the carbon and water it holds, creating a very unstable material (calcium oxide) which needs to hydrate Quicklime will do so very energetically with Quicklime High Calcium Quicklime Pebble and PowderIf a precisely controlled amount of water is added to quicklime, a (violent) reaction ensues with much evolution of heat In the chemical reaction with water the lumps of quicklime break down to a dry fine white powder known as hydrated lime or lime hydrate Quicklime + (a controlled amount of) water > hydrated lime (also called lime hydrate)Application of Quick Lime Hydrated Lime

Lime Production Process and Required Equipment Fote
2023年2月15日 1 In the process of calcination, the granularity of the raw limestone affects the whole process a lot Since the separation of CO2 proceeds slowly from the surface of the limestone to the inside, the calcination of large particle size is more difficult than that of small ones, and it takes a longer timeand what remains is quicklime, calcium oxide Limestone decomposes into quicklime and carbon dioxide: CaCO3> CaO + CO2 by weight 100 > 56 + 44 The process is called ‘calcination’ If calcination is carried out correctly the lumps of quicklime are approximately the same size as the original lumps of limestone but much less dense, becauseApplications of Quicklime Hydrated Lime