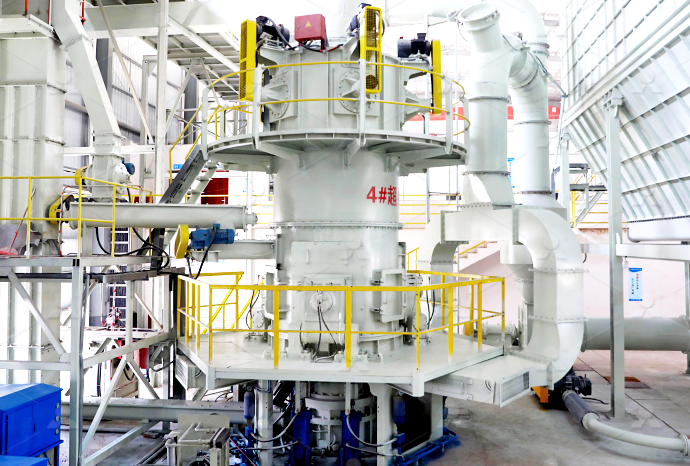
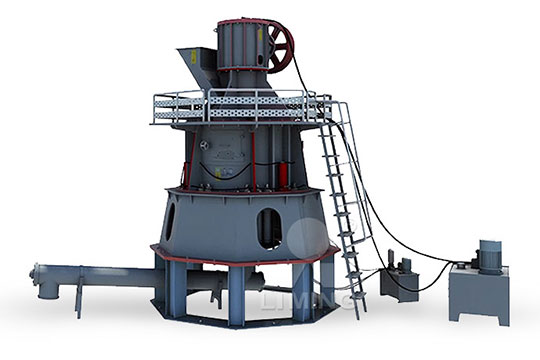
Equipment for burning calcium carbonate from limestone
G[R.jpg)
Lime kiln Wikipedia
A lime kiln is a kiln used for the calcination of limestone (calcium carbonate) to produce the form of lime called quicklime (calcium oxide) The chemical equation for this reaction is CaCO3 + heat → CaO + CO2 This reaction can take place at anywhere above 840 °C (1,540 °F), but is generally considered to occur at 900 °C 展开2022年10月1日 In the lower calcining zone, the calcination continues in a cocurrent flow of the limestone with the combustion gases from the lower burner Fuel injection occurs at the height Decarbonising the lime industry: Stateoftheart ScienceDirect2020年11月29日 Vertical shaft kilns are most efficient kilns found for the calcinations of limestone to produce lime Vertical shaft kiln is a vertical hollow cylindrical furnace fitted with Refractories for Lime Calcination SpringerLink2019年12月10日 Eggshell is a solid waste that is rich in calcium carbonate and can potentially replace limestone for lime production This research examines the environmental impacts of An environmentfriendly process for limestone calcination with

Review—calcination and carbonation of limestone during thermal
2005年11月1日 Because limestone is not pure calcium carbonate, some of the common impurities and ash from a fuel can react to form other calcium compounds (OCCs) when the 2020年10月16日 Here, a novel lime calcination system with carrier gas (CO 2) heating and air cooling is proposed to avoid the mixing problem of the CO 2 and the flue gas This system consists of a new shaft kiln with four processing Novel Lime Calcination System for CO2 Capture and Its 2021年8月24日 The feasibility of a novel method to decarbonise calcium carbonate and coproduce sodium carbonate through simultaneous sequestration of CO 2, and under ambient conditions, is established The core of the Decarbonisation of calcium carbonate at atmospheric This conversion of calcium carbonate to calcium oxide is achieved by heating the limestone to a temperature high enough (eg 1000 C in a lime kiln) to 'drive off' carbon dioxide, CO 2 The HOW TO CALCULATE EFFICIENCY OF YOUR LIME BURNING

Lime and its Production
Limestone (Calcium Carbonate – CaCO3) is burnt in a kiln giving off Carbon Dioxide (CO2) gas and forming Calcium Oxide (CaO) which is commonly known as Quicklime or Lumplime It needs to be burnt at 900°C to ensure a good 2021年1月1日 Lime is a product derived from the thermal decomposition of limestone (mainly calcium carbonate, CaCO3) into quicklime (CaO) and carbon dioxide (CO2), also called calcination Controlled(PDF) Natural and enhanced carbonation of lime in its It is assumed that the limestone extracted contains about 75 wt% calcium carbonate The limestone is then crushed in a jaw crusher and calcined (burned) Process flow diagrams (PFD), equipment list and industrial site configuration Contents in Detail Other Calcium Carbonate Production Cost ReportsCalcium Carbonate Production from Limestone IntratecusThe basic raw material of the cement production is limestone Limestone consist of predominately of calcium carbonate (CaCO 3), in generally its most stable modification known as calcite, in addition, they often contain magnesium, Cement Manufacturing Process INFINITY FOR
.jpg)
grinding equipment for calcium carbonate process CM
27 Sep 2023; Grinding equipment for calcium carbonate is essential in the process of producing fine and ultrafine calcium carbonate powder Calcium carbonate grinding equipment mainly includes ball mill, Raymond mill, vertical roller mill, and ultrafine millThese grinding equipment are widely used in various fields of mining, construction, metallurgy, chemical industry, and so onCalcium carbonate occurs naturally as the principal constituent of limestone, marble and chalk Powdered calcium carbonate is produced by two methods on the industrial scale It is quarried and ground from naturally occurring deposits and in same cases beneficiatedCALCIUM CARBONATE FROM LIMESTONE (PRECIPITATED AND 2017年1月1日 PDF Calcium carbonate (CaCO3) is the most widely used filler material in paper, paint, plastic, food, ceramic, cosmetic, medicine and other Find, read and cite all the research you need on Precipitated Calcium carbonate production, synthesis and properties2021年12月20日 The Importance of Calcium Carbonate Calcium carbonate (CaCO3) comprises more than 4% of the earth’s crust and is found worldwide Its most common natural forms are chalk, limestone, and marble (produced by the sedimentation of small fossilized shellfish, snails, and coral over millions of years)Calcium Carbonate Manufacturing Process and Equipment

Chemicals from Limestone Springer
Chemicals from Limestone The next major raw material for which we discuss the derived chemicals is calcium carbonate, common limestone It is the source of some carbon dioxide, but, more importantly, it is used to make lime (calcium oxide) and slaked lime (calcium hydroxide) Limestone, together with salt and2024年11月4日 The calcium carbonate in the limestone thermally decomposes to form calcium oxide calcium carbonate → calcium oxide + carbon dioxide The calcium oxide formed reacts with the silicon dioxide, which is an impurity in the iron ore, to form calcium silicate This melts and collects as a molten slag floating on top of the molten iron, which is Extraction of iron IGCSE Chemistry Revision Notes Save My Exams2021年1月1日 Lime is a product derived from the thermal decomposition of limestone (mainly calcium carbonate, CaCO3) into quicklime (CaO) and carbon dioxide (CO2), also called calcination(PDF) Natural and enhanced carbonation of lime in its different Calcium carbonate is found naturally in limestone close limestone A type of sedimentary rockWhen limestone is heated strongly, the calcium carbonate it contains absorbs heat (endothermic close Limestone [GCSE Chemistry only] The limestone cycle
.jpg)
Calcium Carbonate (GCC) Hosokawa Alpine
Calcium Carbonate (GCC) produced from chalk, limestone, calcite or marble have developed in recent years from just being a simple cheap filler to highest quality functional additives These demanding requirements are already met 2023年3月28日 Calcination is the hightemperature decomposition of calcium carbonate (CaCO 3) into calcium oxide (CaO) and CO 2 The remaining half of the emissions are caused by fuel combustion, transportation, operation and power consumption A potential approach to reduce CO 2 emissions in the cement industry is to capture it from fluegas systemsExperimental analysis on calcination and carbonation process in calcium 2017年1月1日 The calcium carbonate looping (CaL) process is a promising postcombustion technology for CO2 capture from fossilfired power plants and carbon intense industries like steel and cement manufacturingCalcium Carbonate Looping: CO2 capture by using limestone in 2023年12月7日 The carbon dioxide reacts with the calcium hydroxide to form calcium carbonate Equipment The equipment used in the manufacturing process for calcium carbonate varies depending on the type of calcium carbonate being produced For natural calcium carbonate, the equipment typically includes crusher, screens, grinder mill, and washersWhat is the steps in the Calcium Carbonate Manufacturing
.jpg)
Calcium Carbonate(CaCO3) Limestone Formula, Structure, Uses
2023年12月26日 Chemical Properties of CaCO 3 The chemical properties of calcium carbonate can be visualized in terms of chemical reaction it undergoes Let's have glance on the chemical reactions of CaCO 3 Reaction of CaCO 3 with Hydrochloric Acid (HCl) Calcium Carbonate on reacting with HCl gives calcium chloride salt and carbon dioxide gas which causes effervesencemostly of calcium carbonate (CaCO 3), but to geologists, limestone is only one of several types of “carbonate rocks” These rocks are composed of more than 50% carbonate minerals, generally the minerals calcite (pure CaCO 3) or dolomite (calciummagnesium carbonate, CaMg[CO 3] 2) or both Most carbonate rocks were deposited from seawaterLimestone—A Crucial and Versatile Industrial Mineral Commodity2020年7月30日 In this study, to determine the calcium carbonate availability in eggshells waste and factors those affect its extraction The parameters like temperature, the size of the eggshell powder and the (PDF) Calcium Carbonate Synthesis, Optimization and Characterization The conversion of limestone into quicklime (calcium oxide) and slaked lime (calcium hydroxide Chemguide: Core Chemistry 14 16 Chemically, limestone is calcium carbonate It is a sedimentary rock formed from the shells and skeletons of marine creatures which fell to the bottom of ancient seaslimestone, quicklime and slaked lime chemguide

Precipitated Calcium Carbonate Carmeuse
Calcium carbonate, which is also recognized as the chemical formula CaCO 3, makes up almost five percent of the earth’s crust and is found all throughout the planetCaCO 3 is a combination of CaO (calcium oxide) and CO 2 (carbon dioxide) Calcium carbonate’s most common natural forms are chalk, limestone, and marbleOverview: Calcium carbonate (CaCO 3) is one of the most widely used minerals todayAlso known as limestone, limestone flour, or powdered limestone, it is used in a variety of industrial and commercial applications such as the manufacture of plastics, paints, and adhesives, as well as in environmental applicationsCalcium Carbonate Materials Handled Flexicon Corporation2021年1月21日 This study investigated the performance and process modeling of CO 2 capture through calcium carbonate looping (CCL) using local (Malaysia) limestone as the sorbent The original limestone was compared with two types Frontiers CO2 Capture for Dry Reforming of Natural 2017年3月20日 Limestone is made of the chemical calcium carbonate and was formed by compression of the shells of dead sea creatures at the bottom of clear, tropical seas over millennia It is an excellent building stone , but if heated to a temperature of 600°C it changes to calcium oxide (quick lime), and by mixing this with various other materials and heating, it can Why Burn Limestone? Oldknow’s

Calcium Carbonate Production from Limestone
This report presents a cost analysis of Precipitated Calcium Carbonate (PCC) production from limestone The process examined is a typical calcination process In this analysis, limestone is first calcinated The product reacts with water producing calcium hydroxide, which is further carbonated with carbon dioxide from calcination to produce PCCThe lime cycle shows the stages from quarrying the limestone through to the production of mortars and plasters for our buildings and how it slowly, through the reabsorption of Carbon Dioxide, reverts to its original chemical form (Calcium Carbonate) in the wall Lime burning Limestone (Calcium Carbonate – CaCO3) is burnt in a kiln giving Lime and its ProductionCalcium carbonate is a chemical compound with the chemical formula Ca as an ingredient of cement, or as the starting material for the preparation of builders' lime by burning in a kiln However, because of weathering mainly caused by acid rain, [41] calcium carbonate (in limestone form) is no longer used for building purposes on Calcium carbonate Wikipedia2024年1月19日 We reviewed existing studies of the effects of different calcium carbonate forms Yu, Q L Brouwers, H J H Assessing the chemical involvement of limestone powder in sodium carbonate Maximising the benefits of calcium carbonate in sustainable

Limestone crushing technology and equipment SBM Ultrafine
2023年12月8日 Limestone Properties The main component of limestone is calcium carbonate (CaCO3), with a Mohs hardness of 3 After limestone is mined from limestone, it is crushed to form limestone particles, that is, stone and sand, or further ground to form limestone powder, which is widely used in industries such as building materials, highways, metallurgy, and The calcium carbonate grinding production line is an efficient, stable, and reliable production line with wide application prospects By using advanced equipment and technology, this production line is able to produce highquality calcium carbonate powder to meet the needs of different industries At the same time, the production line also has the advantages of energy Calcium Carbonate Grinding Production Line TAYMACHINERYlimestone burningpdf Free download as PDF File (pdf), Text File (txt) or read online for free This document provides background information on limeburning, including: 1) The chemistry and process of limeburning involves heating limestone (calcium carbonate) to produce quicklime (calcium oxide) and carbon dioxide in a process called calcinationLimestone Burning PDF PDF Carbonate Calcium2024年10月30日 Sedimentary rock Limestone Formation, Calcium Carbonate, Fossils: Limestones originate mainly through the lithification of loose carbonate sediments Modern carbonate sediments are generated in a variety of environments: continental, marine, and transitional, but most are marine The presentday Bahama banks is the best known modern Sedimentary rock Limestone Formation, Calcium Carbonate,
.jpg)
Calcium Oxalate: A Surface Treatment for Limestone
1998年5月1日 Calcium Oxalate: A Surface Treatment for Limestone Tody M Cezar Architectural Stone Carving Course The City and Guilds of London Art School, 124 Kennington Park Road, London, SE11 4DJ, United Kingdom 2005年11月1日 This process can be economical because the raw material is limestone and circulating fluidised beds are suitable process vessels This review will be restricted to limestone, although dolomite CaMg(CO 3) 2 and dolostones, which are mixtures of calcium and magnesium carbonates can also act as sorbents Magnesium carbonate decomposes at a much lower Review—calcination and carbonation of limestone during Limestone is an unusual rock in that it fizzes when dilute acid is placed on its surface It is the presence of calcium carbonate that is responsible for this The calcium carbonate content of limestone rocks has been used from the earliest Limestone, a fizzy rock – introduction — Science 2021年6月3日 Combining carbon dioxide and calcium creates calcium carbonate rocks such as limestone Photo by Freespiritcoast/Alamy Stock Photo Petrifying Climate Change Researchers want to combat climate change by chemically converting carbon dioxide into rock on a Petrifying Climate Change Hakai Magazine

Limestone Geology is the Way
Limestone is a carbonate sedimentary rock that consists predominantly of calcite [CaCO 3]Limestones are the commonest rocks that contain nonsilicate minerals as primary components and, even if they represent only a fraction of all sedimentary rocks (about 20 – 25%), their study is fundamental to understand past environments, climate, and the evolution of lifeLimestone is a very common sedimentary rock consisting of more than 50% calcium carbonate Although it occurs in many different forms, its origins can be traced back to either chemical or biochemical processes that occurred in the geological past, often tens to Limestone origins Science Learning HubLimestone, or calcium carbonate, is the common rock found throughout the world Oldest and perhaps slightly overlooked, limestone is very much part of our everyday life It may be hidden with your walls, in the water you drink, the food you consume, or in the cosmeticsLimestone Formation, Composition, Types and Uses Earth EclipseLimestone is a sedimentary rock composed primarily of calcium carbonate with the occasional presence of magnesium Most limestone is biochemical in origin meaning the calcium carbonate in the stone originated from shelled oceanic creatures Limestone can also be chemical in origin as is the case with travertineLimestone Quarrying and Processing: A LifeCycle Inventory
.jpg)
Calcium Carbonate (Calcite) SpringerLink
2022年4月12日 Limestone is a sedimentary rock comprised chiefly of calcium carbonate (CaCO3) Deposits are extensive around the world Therefore, there is a high variability of limestone deposits Typically, they are formed in two main environments2022年7月11日 Precipitated CaCO3 (PCC) with calcite and aragonite phases have been successfully synthesized using natural limestone Synthesis of PCC was carried out by the simple method of carbonation using (PDF) The Simple Method of Synthesizing Calcite and Aragonite