Where is the calcium carbonate in Acheng produced
.jpg)
Calcium Carbonate CaCO3 CID 10112 PubChem
Ground calcium carbonate (CAS: ) results directly from the mining of limestone The extraction process keeps the carbonate very close to its original state of purity and delivers a Naturally, calcium carbonate is obtained from chalk or limestone It is often known as whiting and is obtained in its natural state with a purity of around 96% Synthetic grades are often known Calcium Carbonate an overview ScienceDirect TopicsCalcium carbonate is divided into two industrial categories: Ground Calcium Carbonate (GCC) and precipitated calcium carbonate (PCC) The two categories use different manufacturing How Calcium Carbonate is Produced?|Manufacturing|CORE2016年12月31日 Calcium carbonate is a chemical compound with the formula CaCO3 formed by three main elements: carbon, oxygen, and calcium It is a common substance found in rocks in (PDF) Calcium Carbonate ResearchGate

Synthesis of precipitated calcium carbonate: a review
2017年3月9日 Precipitated calcium carbonate can be handily and flawlessly produced in a precipitation reaction by reacting aqueous calcium hydroxide, Ca(OH) 2 known as milk of lime 2024年10月26日 Calcium carbonate is either a white powder or a colorless crystal When heated, it produces carbon dioxide and calcium oxide (also called quicklime) Calcium Calcium carbonate Formula, Uses, Names, Facts Britannica2017年1月1日 Calcium carbonate (CaCO3) is the most widely used filler material in paper, paint, plastic, food, ceramic, cosmetic, medicine and other industries In the present paper, Precipitated Calcium carbonate production, synthesis and properties2024年1月19日 Calcium carbonate has three anhydrous crystalline polymorphs, namely vaterite, aragonite, and calcite with increasing thermodynamic stability Amorphous calcium carbonate Maximising the benefits of calcium carbonate in sustainable

Calcium carbonate Wikiwand
Crystal structure of calcite Calcium carbonate shares the typical properties of other carbonatesNotably it reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water:; CaCO 3 (s) + 2 H + (aq) → Ca 2+ (aq) + CO 2 (g) + H 2 O(l) releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above 2017年1月1日 Erdogan and Eken (2017) have also produced precipitated calcium carbonate from waste marble powder by calcinationdissolutionprecipitation method under various conditions of temperatures and timesPrecipitated Calcium carbonate production, Calcium carbonate (CaCO 3) is a substance widely used for various purposes, for example, as a filler and pigment material not only in paper, plastics, rubbers, paints, and inks but also in pharmaceutics, cosmetics, construction materials, and asphalts and as a nutritional supplement in animal foods (1)Besides the socalled ground calcium carbonate (GCC), which is milled from Calcium Carbonate an overview ScienceDirect TopicsCalcium Carbonate (CaCO3)[Limestone] Calcium carbonate is one of the most abundant materials present in nature with the chemical formula CaCO3 Calcium carbonate also called limestone is an example of a metal carbonate used in the Solvay processLimestone: Calcium Carbonate (CaCO3) Uses, Preparation,

Formations of calcium carbonate minerals by bacteria and its
The calcium carbonate (CaCO 3) precipitation process is a straightforward and easily controllable mechanism of MICP that can produce high concentrations of CaCO 3 in short period of time (Dhami et al 2013a)Urease influences the chemical process associated with the formation of biominerals through four different parameters (Hammes and Verstraete 2002) such as pH, 2021年12月20日 The Importance of Calcium Carbonate Calcium carbonate (CaCO3) comprises more than 4% of the earth’s crust and is found worldwide Its most common natural forms are chalk, limestone, and marble (produced by the sedimentation of small fossilized shellfish, snails, and coral over millions of years)Calcium Carbonate Manufacturing Process and EquipmentCalcium carbonate (CaCO 3) produced within coral reefs accounts for more than 25% of the total CaCO 3 buried in marine sediments globally (Jones et al, 2015) Calcium carbonate sediments are highly important in tropical marine environments because they contribute to reef islands, Calcium Carbonate Production, Coral Cover and Diversity along a 2018年5月23日 Calcium carbonate Calcium carbonate, CaCO 3, is one of the most common compounds on Earth, making up about 7% of Earth ’ s crust It occurs in a wide variety of mineral forms, including limestone, marble, travertine, and chalk Calcium carbonate also occurs combined with magnesium as the mineral dolomite, CaMg (CO 3) 2Stalactites and Calcium Carbonate Encyclopedia

Catalytic effect of water on calcium carbonate decomposition
2019年10月1日 Calcium carbonate decomposition was tested in a pure argon Dielectric Barrier Discharge plasma The interaction between plasma and the surface of carbonate increased the decomposition rate due to a thermal effect exclusively, whereas gas phase plasma consecutively dissociated CO 2, producing CO and O 22016年3月1日 Microbially induced calcite precipitation (MICP) refers to the formation of calcium carbonate from a supersaturated solution due to the presence of their microbial cells and biochemical activities (Bosak 2011)During MICP, organisms are able to secrete one or more metabolic products (CO 3 2−) that react with ions (Ca 2+) in the environment resulting in the Formations of calcium carbonate minerals by bacteria and its 2014年5月1日 It also produced the highest 7day UCS of 371 kPa at a calcium carbonate content of 040% Calcium carbonate was precipitated over the entire five meter treatment lengthInfluences of calcium sources on microbially induced 2022年8月29日 Due to the presence of these calcium ions the surface of the oceans are oversaturated with respect to calcium carbonate minerals Importantly, at low to moderate oversaturation levels, and in the presence of Calcium Carbonate Dissolution from the Laboratory to
.jpg)
Calcium carbonate, hydrochloric acid, and their
Spacefilling model of part of the crystal structure of calcium carbonate, CaCO₃ [Wikimedia] CaCO₃ is a widespread compound found in chalk, lime, marble, and more This substance is a crucial pillar of human life – it is used in 2001年1月1日 Calcium carbonate is the main component of chalk, limestone and marble These rocks have a specific behaviour with respect to erosion In contrast to magmatic rocks, they are subject to very Calcium Carbonate: From the Cretaceous Period into the 21st Precipitated Calcium Carbonate (PCC) is a synthetic calcium carbonate produced industrially by means of a recarbonisation process Both GCC or PCC can be used in a wide range of applications For each end use there exists a tailormade product, where fineness and particle size distribution are optimally balanced to meet the technical demands of that particular Calcium Carbonate IMA EuropeMeasurements of hardness are given in terms of the calcium carbonate equivalent (CaCO 3), which is an expression of the concentration of hardness ions in water in terms of their equivalent value of calcium carbonate Water is considered to be hard if it has a hardness of 100 mg/L or more as calcium carbonate Hard water causes bathtub ringsLesson 10: Softening Mountain Empire Community College
.jpg)
experiment 13: calcium carboante Flashcards Quizlet
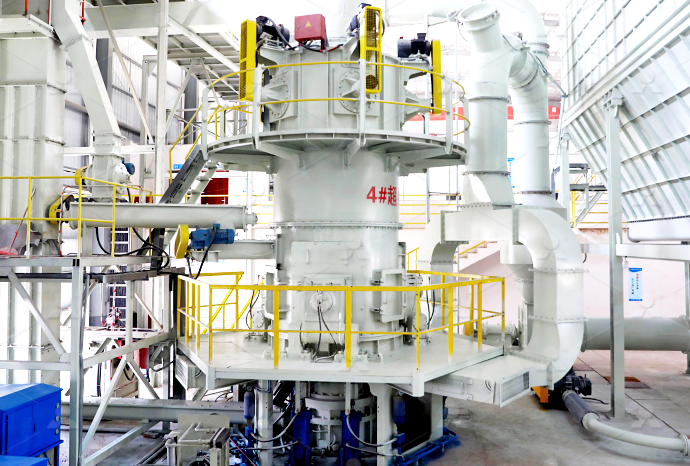
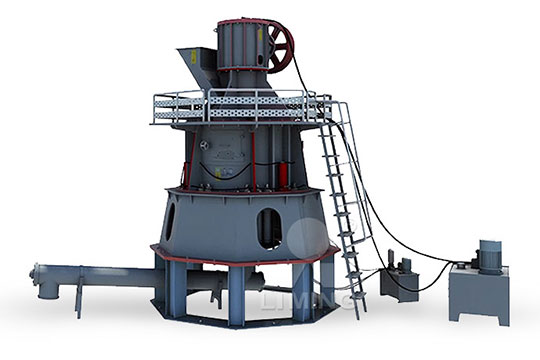
Study with Quizlet and memorize flashcards containing terms like in some solid calcium carbonate sample, calcium bicarbonate, Ca(HCO3)2 is also present write balanced equation for its reaction with hydrochloric acid, How much pure calcium carbonate (10009 g/mol) is required to produce 37 mL carbon dioxide gas according to the reaction used in this experiment? Your unknown 2024年1月19日 Therefore, we posited that diverse calcium carbonate forms can be produced by leveraging various alkaline wastes, offering opportunities for tailored material properties and environmental benefitsMaximising the benefits of calcium carbonate in sustainable 2019年5月11日 Biodeposition of minerals is a widespread phenomenon in the biological world and is mediated by bacteria, fungi, protists, and plants Calcium carbonate is one of those minerals that naturally precipitate as a byproduct of microbial metabolic activities Over recent years, microbially induced calcium carbonate precipitation (MICP) has been proposed as a Microbially induced calcium carbonate precipitation: a 2023年12月7日 The equipment used in the manufacturing process for calcium carbonate varies depending on the type of calcium carbonate being produced For natural calcium carbonate, the equipment typically includes crusher, screens, grinder mill, and washers For synthetic calcium carbonate, the equipment typically includes calciners, dissolvers, and carbonatorsWhat is the steps in the Calcium Carbonate Manufacturing
.jpg)
Phase and morphology of calcium carbonate precipitated by
and concentrations of Ca and carbonate ions, wherein the most common reagents were employed, namely, CaCl 2$2H 2O and NaHCO 3/Na 2CO 3 The initial pH of the carbonate solution was determined by varying the volume ratio of the bicarbonate and carbonate ions Furthermore, for comparison, an excess concentration of carbonate ions was employed to 2021年7月8日 Calcium carbonate and calcium citrate are found in most calcium supplements Learn how to choose which one is right for you Skip to content Menu Health AZ COVID19; Although you can take calcium citrate without Calcium Citrate vs Calcium Carbonate: Which to different calcium carbonate forms”) and present the current research fronts on utilising various alkaline wastes to produce diverse calcium carbonate products in Section “ControllingMaximising the benefits of calcium carbonate in sustainable 2022年9月29日 Method 1 Volume of CO 2 produced Support a gas syringe with a stand, boss and clamp Add 50 cm 3 of dilute hydrochloric acid to a conical flask; Loosely connect the gas syringe Measure 040 g of calcium carbonate; Add the 040 g of calcium carbonate into the conical flask, replace the gas syringe and start the stopwatchRate of Reaction Calcium Carbonate Hydrochloric Acid

Microbially Induced Calcium Carbonate Precipitation in Construction
2020年5月1日 Biocementation or microbially induced calcium carbonate precipitation (MICCP) is a research domain explored by many researchers in the field of civil engineering2024年8月21日 Ground Calcium Carbonate: Produced by crushing and grinding natural limestone, resulting in different particle sizes for various applications Calcination: A critical step where limestone is heated to high temperatures to produce quicklime, which is further processed into calcium oxideThe Ultimate Guide Calcium Carbonate Manufacturing ProcessCalcium carbonate is the principal mineral component of limestone Its chemical and physical properties lie behind the modernday uses of limestone as well as the unique limestone landscapes of the countryside Calcium carbonate – mineral forms The principal mineral component of limestone is a crystalline form of calcium carbonate known as Carbonate chemistry Science Learning Hub2021年12月1日 Coprecipitation of mineralbased salts during scaling remains poorly understood and thermodynamically undefined within the water industry This study focuses on investigating calcium carbonate and calcium sulfate mixed precipitation in scaling Scaling is often observed in the produced water supply as a result of treatment processes Coprecipitation results were CoDeposition Mechanisms of Calcium Sulfate and Calcium Carbonate
.jpg)
Calcium Carbonate in Food Products Periodical by Knowde
How is Calcium Carbonate Produced? Calcium Carbonate is commonly found in the earth’s crust It is available in different forms, such as limestone, marble, calcite, and aragonite The bulk production of Calcium Carbonate occurs by mining or quarrying marble stones Calcium carbonate can naturally be found in shells from shellfish to snails 2020年2月20日 Soil stabilization technology based on microbialinduced carbonate precipitation (MICP) has gained widespread interest in geotechnical engineering MICP has been found to be able to improve soil strength, stiffness, liquefaction resistance, erosion resistance, while maintaining a good permeability simultaneously MICP processes involves a series of Factors affecting the performance of microbialinduced carbonate 2022年3月8日 Calcium Carbonate (CaCO3) is found in limestone, Approximately 8 to 10% of global anthropogenic CO2 emissions are produced by cement industries, and cement production is projected to exceed 6 billion Calcium Carbonate in the Concrete Industry Noah 22 Aqueous Mineralization Process To produce CaCO 3 cement, the carbide lime sludge is first solubilized with aqueous NH 4 Cl then passed through a leaf filter to remove insoluble impurities resulting in an aqueous solution of CaCl 2 and ammonia (NH 3), see Equation (1)NH 3 dissolved in water is in equilibrium with ammonium hydroxide (NH 4 OH) CO 2containing flue gas (11 Calcium Carbonate Cement: A Carbon Capture, Utilization, and

(PDF) Calcium Carbonate ResearchGate
2016年12月31日 Calcium carbonate is a chemical compound with the formula CaCO3 formed by three main elements: carbon, oxygen, and calcium It is a common substance found in rocks in all parts of the world (most 2021年5月10日 Calcium carbonate (CaCO3) minerals secreted by marine organisms are abundant in the ocean These particles settle and the majority dissolves in deeper waters or at the seafloor Dissolution of Calcium carbonate dissolution patterns in the oceanPAPERMAKING Coating MK Joyce, in Encyclopedia of Forest Sciences, 2004 Calcium Carbonate Naturally ground calcium carbonate (GCC) is produced from chalk, limestone, or marble by either a wet or dry grinding process The basic mineral of both chalk and marble is calcite, whose crystals are rhombic in structureCalcium Carbonate an overview ScienceDirect TopicsPrecipitated calcium carbonate is an in organic chemical obtained by calcining naturally occurring lime stone, slaking and carbonation Precipitated calcium carbonate is pure form of lime stone used in the chemical industries Precipitated calcium carbonate (PPC) is used as filler coating pigment for premium quality paper productsPROFILE ON THE PRODUCTION OF CALCIUM CARBONATE AND

Decomposition study of calcium carbonate in cockle shell
2012年2月1日 CaO is normally been produced via thermal decomposition of calcium carbonate (CaCO 3) sources such as limestone which is obtained through mining and quarrying limestone hill2023年11月14日 Calcium carbonate supplements are an effective way to increase your calcium intake if your diet isn’t sufficient, or you have a condition that leads to lower calcium levels Learn about Calcium Carbonate: Uses, Dosage, and Potential Side Effectschanging the calcium sources to Ca(CH 3COO) 2, the samples show a significant improvement in UCS The results of water absorption ratio tests are shown in Fig 2Influences of calcium sources on microbially induced carbonate Particle Morphology Controlling Technique Calcium carbonate has three crystalline polymorphs: calcite, aragonite, and vaterite In the carbonation process, these crystals can be produced by changing various conditions such as concentration and temperature of lime milk, introduction rate of CO 2 gas, and whether or not chemicals are added All reaction formulas are expressed as How Calcium Carbonate is Produced?|Manufacturing|CORE