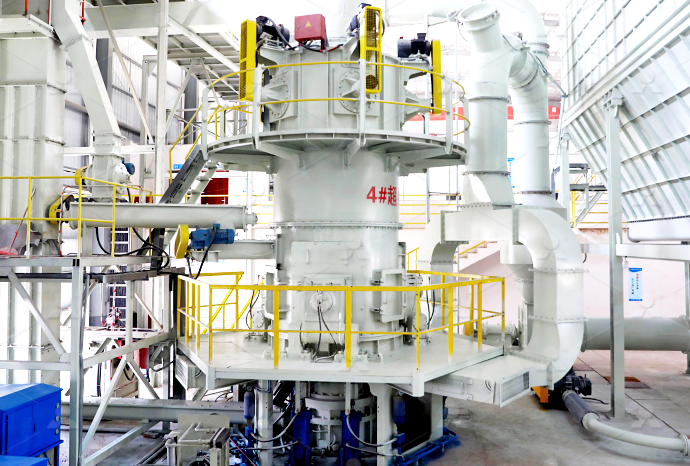
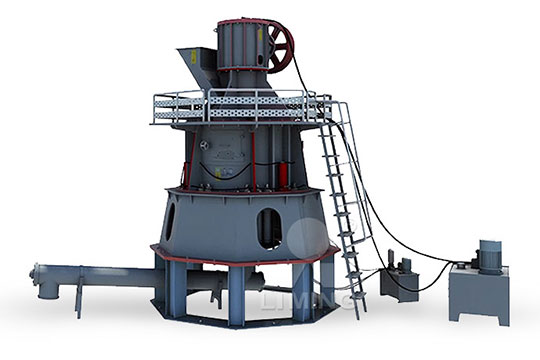
Calcium carbonate grinding system quicklime calcium oxide method

CHEMICAL ANALYSIS OF LIMESTONE/ CALCIUM OXIDE
Conclusions: Three main test methods, ASTM C25, C1271, and C1301, are used for the chemical and physical analysis of limestone and other calciumoxide materials ASTM C25 involves primarilyScope 11 These test methods cover the chemical analysis of highcalcium and dolomitic limestone, quicklime, and hy drated limeStandard Test Methods for Chemical Analysis of Limestone, 2024年4月23日 11 These test methods cover the chemical analysis of highcalcium and dolomitic limestone, quicklime, and hydrated lime These test methods are classified as either Standard Test Methods for Chemical Analysis of Limestone, both quicklime and hydrated lime are slightly soluble in water, but calcium carbonate is hardly soluble at all So washing a small quantity (say 5 to 10 grammes) with excess warm sugar Methods for testing lime in the field Humanitarian Library
.jpg)
ASTM International ASTM C2519 Standard Test
2019年5月1日 11 These test methods cover the chemical analysis of highcalcium and dolomitic limestone, quicklime, and hydrated lime These test methods are classified as either standard 11 These test methods cover the chemical analysis of highcalcium and dolomitic limestone, quicklime, and hydrated lime These test methods are classified as either standard (preferred) ASTMC25 Standard Test Methods for Chemical Analysis of In the present paper a quick thermogravimetric method for the qualitative and quantitative phase analysis of highcalcium limes, enabling the principal components, namely calcium oxide, Phase Analysis of Highcalcium Lime by TG Springer2015年4月1日 This is based on anomalies of oxide solubilites, where electrolysis of molten carbonates form oxides, which precipitate as calcium oxide when mixed with calcium Lime in the limelight ScienceDirect

Soil improvement with quicklime – longtime behaviour and
2018年5月23日 Quicklime consists primarily of calcium oxide, descriptions and requirements are defined in EN 4591 (2010) It improves and solidifies most soil types, the subgrade of traffic 2021年10月4日 Lime is a product derived from the thermal decomposition of limestone (mainly calcium carbonate, CaCO 3) into quicklime (CaO) and carbon dioxide (CO 2), also called Natural and enhanced carbonation of lime in its different 2020年7月30日 In this study, to determine the calcium carbonate availability in eggshells waste and factors those affect its extraction The parameters like temperature, the size of the eggshell powder and the (PDF) Calcium Carbonate Synthesis, Optimization 2024年4月9日 The crystal type can be divided into orthorhombic crystal system and hexagonal system melting point 825 ℃ (decomposition), decomposition of calcium oxide and carbon dioxide One is the hexagonal The raw material for wet grinding calcium by grinding method is heavy calcium carbonate or calcite with whiteness> 93 Calcium carbonate ChemBK
.jpg)
Calcium carbonate Wikipedia
Calcium carbonate shares the typical properties of other carbonatesNotably it reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water:; CaCO 3 (s) + 2 H + (aq) → Ca 2+ (aq) + CO 2 (g) + H 2 If simple, field methods are to be used, it is easier to test the quality of ‘lime’ when it is in the form of quicklime, ie calcium oxide CaO This would be the case if the lime were being bought direct from a kiln before the kiln operator had started to hydrate it Usually, though, as quicklime will deteriorate if left exposed to the airMethods for testing lime in the field Humanitarian Library2024年11月8日 quicklime (CaO), compound of one atom of calcium and one atom of oxygen that is a white or grayish white solid produced in large quantities by roasting calcium carbonate so as to drive off carbon dioxideAt room temperature, CaO will spontaneously absorb carbon dioxide from the atmosphere, reversing the reactionIt will also absorb water, converting itself into Quicklime Formula, Uses, Definition BritannicaCalcium oxide and the specific products derived from it are obtained by the process of calcination of calcium carbonate at high temperature CaO + H2O → Ca(OH)2 + calor Calcium oxide is an alkaline product that allows the regulation of many industrial processes Its principal properties depend on its chemical composition and the thermal treatment that []CALCIUM OXIDE CALCINOR Minerales industriales

Quick Lime Preparation, Properties and Uses Hebei Yayang
2023年10月11日 Quicklime, also referred to as lime (calcium oxide (CaO)), is derived from high quality, natural deposits of limestone (calcium carbonate (CaCO3)) or dolomitic limestone (calcium magnesium carbonate (CaCO3 + MgCO3) Quicklime is produced by heating the stone to almost 2000 degrees Fahrenheit2024年6月23日 Reactivity of QuickLime QuickLime, also known as calcium oxide, exhibits remarkable reactivity due to its chemical composition and properties Understanding the reactivity of QuickLime is crucial in various industrial processes and applications AcidBase Reactions: Quick Lime reacts vigorously with acids to form salts and waterQuickLime 101: Everything About This Super Substance ZMEAs stated previously, the calcium carbonate is heated in the rotary or vertical kilns to drive away CO 2 from limestone CaCO 3 to produce calcium oxide CaO This process is called calcination Calcination conditions highly affect the quality of quicklime CaO that results from this processAn Overview of Lime Slaking and Factors That Affect the Process2023年10月11日 Several companies sell cooking kits using this heating method[12] It is known as a food additive to the FAO as an acidity regulator, a flour treatment agent and as a leavener Calcium carbonate undergoes calcination at temperatures ranging between 1070oC1270oC quicklime (CaO), also called calcium oxide or lime, Calcium oxide Hebei Yayang Spodumene Co, Ltd

Characterization of Calcium Carbonate, Calcium
2009年11月1日 In this paper a complete characterization of lime cycle transitions is described CaCO3 was collected from a Mexican mine and was processed to obtain Ca OH 2 through CaOCalcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO 3; mineral calcite) in a lime kilnThis is accomplished by heating the material to above 825 °C Calcium oxide Wikiwand2024年7月11日 Structure of Calcium Carbonate Calcium carbonate is characterized by its unique chemical structure, represented by the formula CaCO₃At its core, this compound consists of one calcium (Ca) ion bonded to Calcium Carbonate(CaCo₃) Definition, Structure, 2021年12月31日 Grinding aid chemicals which are used in the grinding of calcium carbonate (CaCO3) to prevent agglomeration are chemisorbed on the surfaces of particles, and the compatibility of them with the (PDF) Effects of Grinding Aids Used in Grinding Calcium Carbonate

Limestone [GCSE Chemistry only] The limestone cycle
calcium carbonate → calcium oxide + carbon dioxide Calcium oxide (also known as quicklime) is a key ingredient in the making of cement and is also used to make certain types of plasterAt 1200K, calcium carbonate decomposes to give carbon dioxide and calcium oxide CaCO 3 → CaO + CO 2; On reacting with dilute acids, calcium carbonate gives carbon dioxide CaCO 3 + 2HC l → CaCl 2 + H 2 O +CO 2; Application of Calcium Carbonate Calcium carbonate is largely employed in the pulp and paper industryLimestone: Calcium Carbonate (CaCO3) Uses, Preparation, Several companies sell cooking kits using this heating method[12] It is known as a food additive to the FAO as an acidity regulator, a flour treatment agent and as a leavener Calcium carbonate undergoes calcination at temperatures ranging between 1070oC1270oC quicklime (CaO), also called calcium oxide or lime, Calcium oxide2017年1月3日 Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate ($\ce{CaCO3}$; mineral calcite) in a lime kiln This is accomplished by heating the material to above 825 °C (1,517 °F), a process called calcination or limeburning, to liberate a molecule of carbon dioxide ($\ce{CO2}$), How is the process of converting calcium carbonate (CaCO3) into calcium
.jpg)
Standard Test Methods for Chemical Analysis of Limestone, Quicklime
2024年4月23日 13 Alternative or optional test methods are provided for those who wish to use procedures shorter or more convenient than the standard methods for the routine determinations of certain constituents Optional test methods may sometimes be preferred to the standard test methods, but frequently the use of modern and expensive instrumentation is indicated which 2017年8月16日 Section Aluminum Oxide 15 Available Lime Index 28 Calcium and Magnesium Oxide: Alternative EDTA Titration Method 31 Calcium Carbonate Equivalent 33 Calcium Oxide: Gravimetric Method 16 Volumetric Method 17 Carbon Dioxide by Standard Method 22 Combined Oxides of Iron and Aluminum 12 Ferrous Iron X5 Free Calcium Oxide X6 Free Moisture in Standard Test Methods for Chemical Analysis of Limestone, Quicklime Our calcined products are produced in energy efficient regenerative kilns where Calcium Oxide (quicklime) is obtained The later grinding and screening process generates the different calcium oxide products from granular products to highly reactive micronized lime Calcium Oxide (quicklime) can be delivered in bags, big bags or bulk Granular Pulverized 0 – 2 mm Calcium Oxide (quicklime) Cales de LliercaLime, also known as calcium oxide, appears in lumpy or granular form, possesses high density, and is sparingly soluble in water, being an alkaline substance It absorbs moisture from the air, transforming into either calcium hydroxide or Quicklime (Calcium Oxide) APEX TW

Guide to Calcium Carbonate Grinding: Mills, Tips, and
2023年6月25日 Crushing: The calcium carbonate stones just mined from the quarry are relatively large, and they need to be crushed by a jaw crusher and a hammer crusher in turn to the feed fineness (10mm20mm) that can enter the methods must be left to the discretion of each laboratory 14 The analytical procedures appear in the following order: Section Aluminum Oxide 15 Available Lime Index 28 Calcium and Magnesium Oxide: Alternative EDTA Titration Method 31 Calcium Carbonate Equivalent 33 Calcium Oxide: Gravimetric Method 16 Volumetric Method 17Standard Test Methods for Chemical Analysis of Limestone, Quicklime Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kilnThis is accomplished by heating the material to above 825 °C (1,517 °F), a process called calcination or limeburning, to liberate a molecule of carbon dioxide (CO2), leaving quicklimeCalcium Oxide Limeco2021年1月1日 Lime is a product derived from the thermal decomposition of limestone (mainly calcium carbonate, CaCO3) into quicklime (CaO) and carbon dioxide (CO2), also called calcination(PDF) Natural and enhanced carbonation of lime in its different

The Processing of Calcium Rich Agricultural and Industrial
2021年5月1日 Steps involved in the synthesis of calcium carbonate nanoparticles from cockle shells Islam et al (2012) reported an easy, costeffective, and novel method for the synthesis of CCPs (aragonite Quicklime called Calcium Oxide is created to discharge carbon dioxide through calcinating calcium carbonate (limestone)Quicklime is also called handpicked lime, burnt lime, lump lime, calcining lime, and caustic lime It is an acidic material; by burning calcium carbonate limestone at approximately 900 degrees celsius, carbon dioxide is placed at this high temperature, and the All about Quicklime Calcium Oxide – NBN MineralsCalcium oxide (CaO), also known as quicklime or burnt lime, A value that indicates the relative concentrations of products and reactants in an equilibrium system Carbon A reaction vessel contains some amount of calcium carbonate and calcium oxide, and the system is found to be at equilibrium If carbon dioxide is injected into the Calcium Oxide (AP Chemistry) Vocab, Definition, Explanations2017年3月3日 PDF Beneficiation and Mineral Processing of Calcium Carbonate and Calcium Sulphate Find, read and cite all the research you need on ResearchGateBeneficiation and Mineral Processing of Calcium Carbonate and Calcium

Quicklime – Saudi Lime
Quick Lime is the result of heating the high purity Limestone (9799% CaCO3), after being crushed to suitable particle size in vertical Kilns and applying a high temperature running between 1100°C to 1300°C Through this operation Calcium Carbonate decomposes to high purity Calcium oxide (Quick Lime) and Carbon dioxide gasCalcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO 3; mineral calcite) in a lime kilnThis is accomplished by heating the material to above 825 °C (1,517 °F), [6] [7] a process called calcination or limeburning, to liberate a molecule of carbon dioxide (CO 2), leaving quicklime Calcium oxide Wikiwand / articles2019年6月7日 The solgel technique has many advantages over the other mechanism for synthesizing metal oxide nanoparticles such as being simple, cheap and having low temperature and pressure Utilization of waste materials Synthesis of NanoCalcium Oxide from Waste 2024年11月13日 CaO + H 2 O → Ca(OH) 2 This reaction is very exothermic and results in the creation of large clouds of steam Preparation of Calcium Oxide (quick lime) Calcium oxide can be made in a lime kiln by thermal breakdown of calcium carbonate (CaCO 3; mineral calcite)containing materials like limestone or seashells Calcination is the term for the process of Calcium Oxide Overview, Structure, Formula, Examples, Uses, FAQs

Differences Between Hydrated Lime and Quicklime
2023年11月9日 Burning (calcination) of calcium carbonate in a lime kiln above 900 °C (1,650 °F)[4] converts it into the highly caustic material burnt lime, unslaked lime or quicklime (calcium oxide) and, through subsequent addition of water, into the less caustic (but still strongly alkaline) slaked lime or hydrated lime (calcium hydroxide, Ca(OH)2), the process of which is called 2024年7月30日 Common Name of Calcium Oxide is Quicklime Calcium Oxide Chemical Formula Chemical Formula of calcium oxide is CaO Calcium Oxide Molecular Weight Molecular weight of calcium oxide is approximately 5608 amu Molecular Weight of Calcium Oxide can be obtained as Atomic weight of calcium is approximately 4008 grams per mole (g/mol)Calcium Oxide Formula, Properties, Preparation and UsesThe root of the word calcination refers to its most prominent use, which is to remove carbon from limestone (calcium carbonate) through combustion to yield calcium oxide (quicklime) This calcination reaction is CaCO 3 (s) → CaO(s) + CO 2 (g) Calcium oxide is a crucial ingredient in modern cement, and is also used as a chemical flux in smeltingCalcination WikipediaSection Aluminum Oxide 15 Available Lime Index 28 Calcium and Magnesium Oxide: Alternative EDTA Titration Method 31 Calcium Carbonate Equivalent 33 Calcium Oxide: Gravimetric Method 16 Volumetric Method 17 Carbon Dioxide by Standard Method 22 Combined Oxides of Iron and Aluminum 12 Ferrous Iron X5 Free Calcium Oxide X6 Free Moisture in Hydrated Lime 21 Free ASTMC25 Standard Test Methods for Chemical Analysis of
.jpg)
Calcium oxide Wikipedia
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound It is a white, caustic , alkaline , crystalline solid at room temperature The broadly used term lime connotes calciumcontaining inorganic compounds , in which carbonates , oxides , and hydroxides of calcium, silicon , magnesium , aluminium , and iron predominateThe crushed small pieces of quicklime are sent to the silo by the elevator, and then sent to the mill grinding chamber for grinding evenly and quantitatively by the vibrating feederFine powder processing can choose LM vertical mill, MTW European mill, 5X European intelligent mill; The ultrafine powder processing generally adopts LUM ultrafine vertical mill, MW ring roller micro quicklime processing plant, quicklime grinding machine, quicklime